This is a prescription-only medicine (POM).
A valid prescription is required to purchase this product.
Don’t have a prescription? Our team of vets is here to help.
Add to cart as normal and follow the steps. Available in Ireland only.
Size:
Eprecis Injection for Beef and Dairy Cattle is used for the treatment of and control of gastro-intestinal worms (including inhibited Ostrtagia ostertagia and Cooperia spp.), lungworms, warbles, sucking lice, chorioptic and sarcoptic mange mites in beef and dairy cattle. Eprecis Injection has no withdrawl time for dairy cattle. The Herd pack contains 2 X 250ml bottles, 1 X 100ml bottle plus a free injector.
Active Ingredient: Eprinomectin
Target Species: Cattle
Administration Method: Subcutaneous Injection
Treats and Controls: Gastro-intestinal worm, lungworm, sucking and biting lice and mange mites
Withdrawal Time: 63 days for cattle intended for meat and offal, there is no withdrawal time for cattle producing milk for human consumption.
Dosage: 1 ml per 100 kg of bodyweight.
| Body Weight | Dose Volume | Number of full doses per pack: | ||
| 100ml | 250ml | 600ml | ||
| 100kg | 1 ml | 100 | 250 | 600 |
| 200kg | 2 ml | 50 | 125 | 300 |
| 300kg | 3 ml | 33 | 83 | 200 |
| 400kg | 4 ml | 25 | 62 | 150 |
| 500kg | 5 ml | 20 | 50 | 120 |
| 600kg | 6 ml | 16 | 41 | 100 |
| 700kg | 7 ml | 17 | 35 | 85 |
Always read the label and all enclosed information for Eprecis Injection before administering to animals!
This product is only licensed for sale within the Republic of Ireland
Click here to Download Data Sheet
Health Products Regulatory Authority
Summary of Product Characteristics
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Eprecis 20 mg/ml solution for injection for cattle, sheep and goats
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml contains:
Active substance:
Eprinomectin 20.0 mg
Excipients:
Butylhydroxytoluene (E321) 0.8 mg
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection.
Clear colourless to pale yellow solution.
4 CLINICAL PARTICULARS
4.1 Target Species
Cattle, sheep and goats.
4.2 Indications for use, specifying the target species
Treatment of infestations by the following internal and external parasites sensitive to eprinomectin:
Cattle Adult
L4 Inhibited L4
Gastrointestinal roundworms
Ostertagia ostertagi
Ostertagia lyrata
Ostertagia spp.
Cooperia oncophora
Cooperia pectinata
Cooperia surnabada
Cooperia punctata
Cooperia spp.
Haemonchus placei
Trichostrongylus axei
Trichostrongylus colubriformis
Trichostrongylus spp.
Bunostomun phlebotomum
Nematodirus helvetianus
Oesophagostomum radiatum
Oesophagostomum spp.
Trichuris spp.
Lungworms
Dictyocaulus viviparus
Sucking lice:
Haematopinus eurysternus,
Linognathus vituli,
Solenopotes capillatus
Horn flies:
Haematobia irritans
Warbles (parasitic stages):
Hypoderma bovis,
Hypoderma lineatum
Mange mites:
Sarcoptes scabiei var. bovis
Prevention of reinfestations:
The product protects treated animals against reinfestations with:
Trichostrongylus spp. (including Trichostrongylus axei and Trichostrongylus colubriformis), Haemonchus placei, Cooperia spp. (including Cooperia oncophora, Cooperia punctata, Cooperia surnabada), Dictyocaulus viviparus, Oesophagostomum radiatum, Ostertagia spp.(including Ostertagia ostertagi and Ostertagia lyrata) and Nematodirus helvetianus for 14 days.
Haematobia irritans for at least 7 days.
Sheep
Gastrointestinal roundworms
(adult) Teladorsagia circumcincta (pinnata/trifurcata),
Haemonchus contortus
Trichostrongylus axei
Trichostrongylus colubriformis
Nematodirus battus
Cooperia curticei
Chabertia ovina
Oesophagostomum venulosum
Lungworm (adult)
Dictyocaulus filaria
Goats
Gastrointestinal roundworms (adult)
Teladorsagia circumcincta (pinnata/trifurcata)
Haemonchus contortus
Trichostrongylus axei
Trichostrongylus colubriformis
Nematodirus battus
Cooperia curticei
Oesophagotomum venulosum
Lungworm (adult)Dictyocaulus filaria
4.3 Contraindications
Do not use in other animal species.
Do not use in known cases of hypersensitivity to the active substance or to any of the excipients.
Do not administer orally or by intramuscular or by intravenous injection.
4.4 Special warnings for each target species
Cattle, sheep and goats
Care should be taken to avoid the following practices because they increase the risk of development of resistance and could ultimately result in ineffective therapy:
Too frequent and repeated use of anthelmintics from the same class, over an extended period of time.
Underdosing, which may be due to underestimation of bodyweight, misadministration of the product, or lack of calibration of the dosing device (if any).
Suspected clinical cases of resistance to anthelmintics should be further investigated using appropriate tests (e.g. Faecal Egg Count Reduction Test).
Where the results of the test(s) strongly suggest resistance to a particular anthelmintic, an anthelmintic belonging to another pharmacological class and having a different mode of action should be used.
If there is a risk for re-infection, the advice of a veterinarian should be sought regarding the need for and frequency of repeat administration.
Cattle
Resistance to other macrocyclic lactones has been reported in parasite species in cattle within the EU.
Therefore, use of this product should be based on local (regional, farm) epidemiological information about susceptibility of nematodes and recommendations on how to limit further selection for resistance to anthelmintics.
Sheep and goats
Resistance to eprinomectin in parasite species in goats and sheep has been reported within the EU.
Therefore, use of this product should be based on local (regional, farm) epidemiological information about susceptibility of nematodes and recommendations on how to limit further selection for resistance to anthelmintics.
4.5 Special precautions for use Special precautions for use in animals
Usual aseptic procedures for administration of a parenteral injection should be followed.
Not to be used in other species; avermectins can cause fatalities in dogs, especially Collies, Old English Sheepdogs and related breeds and crosses, and also in turtles/tortoises.
The death of warble fly larvae in the oesophagus or spinal cord canal may lead to secondary reactions.
In order to avoid secondary reactions due to the death of Hypoderma larvae in the oesophagus or the spine, it is recommended to administer the product at the end of the period of fly activity and before the larvae reach their resting site.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
People with known hypersensitivity to eprinomectin or to any of the excipients should avoid contact with the veterinary medicinal product
The veterinary medicinal product causes serious eye irritation.
Avoid contact with the eyes. Wash any splashes from eyes immediately with water.
This product may cause neurotoxicity.
Care should be taken when handling the product to avoid self-injection. In case of accidental injection, seek medical advice immediately and show the package leaflet or the label to the physician.
Avoid contact with the skin.
Wash any splashes from skin immediately with water.
Avoid oral exposure.
Do not eat, drink or smoke while handling the veterinary medicinal product.
Wash hands after use. T
he excipient glycerol formal may cause harm to the unborn child.
In addition, the active substance eprinomectin can be transferred to breast milk. Pregnant/breast-feeding women and women of childbearing age should therefore avoid exposure to this product.
Other precautions
Eprinomectin is very toxic to dung fauna and aquatic organisms, is persistent in soils and may accumulate in sediments.
The risk to aquatic ecosystems and dung fauna can be reduced by avoiding too frequent and repeated use of eprinomectin (and products of the same anthelmintic class) in cattle, sheep and goats.
The risk to aquatic ecosystems will be further reduced by keeping treated cattle, sheep and goats away from water bodies for two to five weeks after treatment.
4.6 Adverse reactions (frequency and seriousness)
Cattle:
Following treatment, moderate to severe swelling at the site of injection is very common.
Typically, the swelling resolves within 7 days, but induration (hardness) may persist for in excess of 21 days.
Swelling may be associated with mild to moderate pain. This reaction disappears without any treatment and does not impair the safety or efficacy of the veterinary medicinal product.
Sheep and goats:
Slight to moderate swelling at the injection site is very common.
Typically the swelling resolves within 16 to 18 days.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s))
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports)
4.7 Use during pregnancy, lactation or lay
Cattle:
Can be used during pregnancy and lactation.
Sheep and goats:
The safety of eprinomectin during pregnancy in sheep and goats has not been tested. Use only according to the benefit/risk assessment of the responsible veterinarian in these species.
4.8 Interaction with other medicinal products and other forms of interactions
Since eprinomectin binds strongly to plasma proteins, this should be taken into account if it is used in association with other molecules having the same characteristics.
4.9 Amounts to be administered and administration route
Subcutaneous use.
For single administration only.
Administration of 0.2 mg of eprinomectin per kg bodyweight; corresponding to 0.1ml of the veterinary medicinal product per 10 kg bodyweight.
In goats, the volume per injection site should not exceed 0.6 ml. 50 ml and 100 ml vials
Do not exceed 30 broachings per vial.
If more than 30 broachings are required, use of a draw off needle is recommended.
250 ml and 500 ml vials
Do not exceed 20 broachings per vial.
If more than 20 broachings are required, use of a draw off needle is recommended.
To ensure administration of a correct dose, bodyweight should be determined as accurately as possible and accuracy of the dosing device should be checked.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Cattle, sheep:
After subcutaneous administration of up to 5 times the recommended dose, no adverse events were observed except a transient reaction (swelling followed by induration) at the injection site.
The safety of the product in goats has not been demonstrated in overdose studies.
4.11 Withdrawal period(s)
Cattle:
- Meat and offal: 63 days
- Milk: zero hours.
Sheep:
- Meat and offal: 42 days
- Milk: zero hours.
Goats:
- Meat and offal: 42 days
- Milk: zero hours.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
Pharmacotherapeutic group:
endectocides, macrocyclic lactones, avermectins.
ATC vet code:
QP54AA04
5.1 Pharmacodynamic properties
Eprinomectin is a member of the macrocyclic lactone class of endectocides.
Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve or muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite.
Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated chloride channels; the macrocyclic lactones have a low affinity for other mammalian ligand-gated chloride channels, and they do not readily cross the blood-brain barrier.
5.2 Pharmacokinetic particulars
Absorption In cattle,
following subcutaneous administration, the bioavailability of eprinomectin is about 89%.
The maximal mean plasma concentration of 58 µg/L was reached after 36-48 h.
In lactating sheep,
the maximal mean plasma concentration of 19.5 µg/L was reached 33.6 hours after subcutaneous administration.
The area under the curve mean value over a period of 7 days after dose injection was 73.3 µg*day/L.
In non-lactating sheep,
the maximal mean plasma concentration of 11.3 µg/L was reached after 26.7 hours after dose administration.
The area under the curve mean value over a period of 7 days after treatment was 42.5 µg*day/L
In goats,
the maximal mean plasma concentration of 20.7 µg/L was reached 36 h after administration.
The area under the curve mean value over a period of 7 days was 66.8 µg*day/L.
Distribution
There is a linear relationship between the dose administered and plasma concentration observed in the therapeutic dose range from 0.1 to 0.4 mg/kg. Eprinomectin is highly bound (greater than 99%) to plasma proteins.
Metabolism
Eprinomectin is not extensively metabolised.
Metabolites amount to approximately 10% of the total residues in plasma, milk, edible tissues and faeces.
Elimination
In cattle, eprinomectin is eliminated with a half-life of 65-75 h and the major route of elimination is via faeces.
In sheep, eprinomectin is eliminated with a comparable half-life of 62-78 h.
In goats, eprinomectin is eliminated with a half-life of 91 hours.
Environmental properties Like other macrocyclic lactones, eprinomectin has the potential to adversely affect non-target organisms.
Following treatment, excretion of potentially toxic levels of eprinomectin may take place over a period of several weeks.
Faeces containing eprinomectin excreted onto pasture by treated animals may reduce the abundance of dung feeding organisms which may impact on the dung degradation.
Eprinomectin is very toxic to dung fauna and aquatic organisms, is persistent in soils and may accumulate in sediments.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Butylhydroxytoluene (E321)
Dimethyl sulfoxide
Glycerol formal stabilised
6.2 Major incompatibilities
In the absence of compatibility studies, this veterinary medicinal product must not be mixed with other veterinary medicinal products.
6.3 Shelf-life
Shelf life of the veterinary medicinal product as packaged for sale: 3 years.
Shelf life after first opening the immediate packaging: 6 months.
6.4 Special precautions for storage
This veterinary medicinal product does not require any special storage conditions.
6.5 Nature and composition of immediate packaging
Nature of immediate packaging: Amber multilayer plastic vials (polypropylene / ethylene vinyl alcohol / polypropylene) with bromobutyl rubber stoppers and aluminium caps and plastic flip-off discs in a cardboard box.
Pack sizes:
50 ml vial
100 ml vial
250 ml vial
500 ml vial
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal products or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
Extremely dangerous to fish and aquatic life.
Do not contaminate ponds, waterways or ditches with the veterinary medicinal product or empty container.
7 MARKETING AUTHORISATION HOLDER
Ceva Santé Animale
10, avenue de La Ballastière
33500 Libourne
France
8 MARKETING AUTHORISATION NUMBER(S)
VPA10815/024/001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 11 September 2015
Date of last renewal: 25 August 2020
10 DATE OF REVISION OF THE TEXT
July 2021
Cattle Injectables
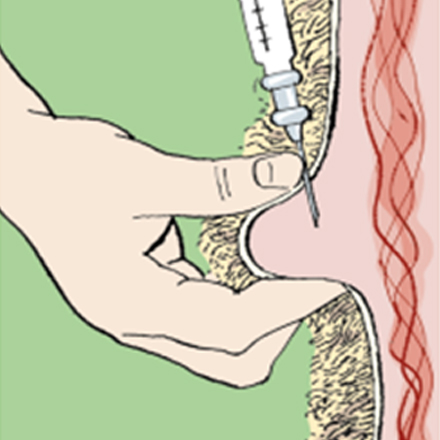
Injectables should be given according to the manufacturer’s instructions at the recommended injection site.
• Always use a clean, sterile syringe and needle. If using a multiple injection gun, ensure the needle is disinfected between injections, e.g. with an automatic sterilisation system.
• If the site to be injected is dirty, clean the skin and swab with an alcohol-impregnated wipe or cotton wool.
• Before injecting, check the expiry date and read the instructions of the product to be used. Some products need to be shaken before use.
• Use the correct-sized needle according to the size of the animal and site of injection.
• Ensure the animal is adequately restrained before attempting the injection.
• Take care to ensure it is given subcutaneously and not intramuscularly. Raise a fold of skin at the injection site (mainly neck but some are ear) recommended by the product manufacturer and inject carefully into the space created.
• If a large dose is to be delivered, it may be advisable to split the dose between two injection sites. After the injection, briefly massage the site to improve the dispersal of the injected material.
• Dispose of the needle and syringe in appropriate clinical waste and sharps containers.
What is Eprinomectin?
Eprinomectin is a semi-synthetic compound of the avermectin family, intended for the treatment of internal and external parasites in cattle and in lactating cows.
Eprinomectin is a mixture of two homologues, eprinomectin B1a (90%) and eprinomectin B1b (10%), which differ by a methylene group in the C25.
The recommended dosage regimen is a single dose of 0.5 mg/kg bw (0.1 ml/10 kg bw) applied topically along the midline of the animal's back.
How does Eprinomectin work?
The precise mode of action of eprinomectin remains unknown, despite extensive investigations with a variety of compounds from the same class.
Eprecis Injection for Beef and Dairy Cattle is used for the treatment of and control of gastro-intestinal worms (including inhibited Ostrtagia ostertagia and Cooperia spp.), lungworms, warbles, sucking lice, chorioptic and sarcoptic mange mites in beef and dairy cattle. Eprecis Injection has no withdrawl time for dairy cattle.
Active Ingredient: Eprinomectin
Target Species: Cattle
Treats and Controls: Gastro-intestinal worm, lungworm, sucking and biting lice and mange mites
Withdrawal Time: 63 days for cattle intended for meat and offal, there is no withdrawal time for cattle producing milk for human consumption
Legal Status: POM
Here at Agridirect we have joined forces with DPD to ensure all packages are delivered promptly and safely to you. We ship to all mainland countries within the EU. Deliveries take place Monday to Friday excluding bank holidays. Once your order has been dispatched from our warehouse you will be notified by email. If there is a delay with your order for any reason you will be contacted immediately.
Due to Brexit we are temporarily unable to ship to the UK. Shipping to Northern Ireland will remain in place.
| Ireland (ROI & NI) | EU (Mainland Only) |
| 2-4 Working Days | 4-6 Working Days |
Some products have an extended delivery time, this is noted on the products.
| Country | Orders Under €100 | Orders Over €100 |
| Ireland (ROI & NI) | €7.99 | €0.00 |
| Austria | €39.99 | €32.00 |
| Belgium | €36.99 | €29.00 |
| Czech Republic | €39.99 | €32.00 |
| Denmark | €39.99 | €32.00 |
| Finland | €51.99 | €45.00 |
| France | €36.99 | €29.00 |
| Germany | €36.99 | €29.00 |
| Hungary | €43.99 | €37.00 |
| Italy | €49.99 | €42.00 |
| Luxenburg | €36.99 | €29.00 |
| Netherlands | €36.99 | €29.00 |
| Poland | €36.99 | €29.00 |
| Portugal | €51.99 | €45.00 |
| Slovakia | €43.99 | €37.00 |
| Slovenia | €43.99 | €37.00 |
| Spain | €49.99 | €42.00 |
| Sweden | €49.99 | €42.00 |
A selection of the products we sell are only licensed for sale within the Republic Of Ireland and can not be shipped outside of the country. These products are noted as only being available within the Republic of Ireland on the individual product pages.
There may be an addition charge on certain bulky items. This charge will be clearly marked on an applicable products and will be explained on the checkout page before payment has been made.
We’re sorry your purchase didn’t work out. But don’t worry; we have a great returns policy to help you out.
All purchases can be returned to us within 14 days of delivery and returned goods must be received within 14 days from the date you informed us of the return.
Purchases may be opened for inspection but must not be used and must be repackaged securely in the original packaging if you wish to return it.
If we discover goods have been used or there has been a loss in value of the goods due to damage to the goods, while in your care or whilst being returned to us, we will reduce the amount refunded, which may amount to the full cost of the product, to cover loss of value of goods.
All returns should be complete which includes boxes, manuals and accessories that may have been included with the order.
All returns must be packaged appropriately for shipping, we will not accept responsibility for damages or loss which occur during shipping of a return product.
We accept no responsibility for goods damaged or lost while in transit to us.
We have partnered with DPD to make your returns process easy and secure. simply follow the steps below and bring your package to an official DPD pickup point.
1) All returns must be accompanied with a fully filled out returns form which can be downloaded returns form PDF.
2) To print off your return label click DPD Returns page or visit www.dpd.ie/returns and follow the on-screen instructions. Make sure and use your order number as your reference.
3) Bring your package to a DPD pickup point. To find your nearest drop off point DPD pickup shops.
Once the returned product has been received into our warehouse and been fully inspected a refund will be issued.
If you choose not to use the DPD returns service we recommend that you use a method that can be tracked.
For the return of bulk products please contact us at sales@agridirect.ie
First off, if you have received a damaged electrical product from us, do not plug it in. Any electrical products that are plugged in are deemed ‘as used and accepted’ and are not accepted as returns. All damages must be reported to us via phone or email within 24hours of receipt of goods. Please ensure you check your items upon delivery.
How do I begin the returns process?
If you wish to begin the return process, please email us at sales@agridirect.ie and ensure the following information is included in your email. Your name, phone number, Order id, the item you wish to return, reason for return and if the product is damaged we require photos of the product.
Once you have sent us all required information a member of our team will assess your claim and will contact you as soon as possible. Please hold off on returning products until a member of our team has called you to confirm.
Once the returned product has been returned to us and fully inspected a refund will be issued.
Please Note: A typical timeline for a refund to show in your account is up to 10 working days from the date processed, depending on your bank.
Would you like to send this voucher to the recipient via email?
Yes No