This is a prescription-only medicine (POM).
A valid prescription is required to purchase this product.
Don’t have a prescription? Our team of vets is here to help.
Add to cart as normal and follow the steps. Available in Ireland only.
Volume:
Ivomec Super injection is a broad spectrum anti parasitic for cattle. It is used for the treatment and control of gastro-intestinal nematodes, lungworms, adult liver fluke, eyeworms, warbles, mites and lice in beef and non lactating dairy cattle. Treating for these parasites has dramatic economic benefits, as it helps to maintain peak physical health and reduce animal fattening times.
Beef cattle were housed and treated with either Ivomec Super or Ivomec Classic injection plus TRODAX (nitroxynil), or Triclabendazole (TCBZ) drench, or Ivomec Classic injection only (no flukicide). There were no significant differences in liveweight gain between the different flukicide groups and no cattle developed clinical signs of fluke infection. Flukicide treatment of animals that had +ve fluke egg counts at housing resulted in significantly higher weight gain over the 112-day trial period compared with cattle that were not treated.
This led to the conclusion that treating animals at risk of fluke at the point of housing will ensure that animals benefit immediately from the production improvements associated with fluke control.
(Data courtesy of Boehringer Ingelheim).
Active Ingredient: Ivermectin, Clorsulan
Target Species: Cattle
Administration Method: Subcutaneous injection (under the skin)
Treats and Controls: Gastro-intestinal roundworms, lungworms, adult liver fluke, eyeworms, warbles, mites and lice
Withdrawal Time: 66 days for animals intended for meat and offal, Not permitted for use on animals producing milk for human consumption.
Dosage: 1 ml per 50 kg of bodyweight
| Body Weight | Dose Volume | Number of full doses per pack: | ||
| 50ml | 200ml | 500ml | ||
| 50kg | 1 ml | 50 | 200 | 500 |
| 100kg | 2 ml | 25 | 100 | 250 |
| 150kg | 3 ml | 16 | 66 | 166 |
| 200kg | 4 ml | 12 | 50 | 125 |
| 250kg | 5 ml | 10 | 40 | 100 |
| 300kg | 6 ml | 8 | 33 | 83 |
| 350kg | 7 ml | 7 | 28 | 71 |
| 400kg | 8 ml | 6 | 25 | 62 |
| 450kg | 9 ml | 5 | 22 | 55 |
| 500kg | 10 ml | 5 | 20 | 50 |
| 550kg | 11 ml | 4 | 18 | 45 |
| 600kg | 12 ml | 4 | 16 | 41 |
Always read the label and all enclosed information for Ivomec Super Injection before administering to animals!
When cattle have to graze on pasture contaminated with infective larvae of cattle nematodes, treatment with Ivomec Super Injection for Cattle at the recommended dose rate can control re-infection with Haemonchus placei and Cooperia spp., acquired up to 14 days after treatment, Ostertagia ostertagi and Oesophagostomum radiatum acquired up to 21 days after treatment and Dictyocaulus viviparus acquired up to 28 days after treatment.
To obtain optimal benefit from the persistent activity of Ivomec Super Injection for Cattle in grazing animals, it is recommended that calves which are set-stocked in the first grazing season should be treated 3, 8 and 13 weeks after the day of turn-out. This can protect the animals from parasitic gastro-enteritis and lungworm disease throughout the grazing season, provided they are set-stocked, all the calves are included in the programme and that no untreated cattle are added to the pasture. Treated animals should always be monitored according to good husbandry practices.
This product is only licensed for sale within the Republic of Ireland
Click here to Download Data Sheet
Summary of Product Characteristics
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Ivomec Super Injection for Cattle
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection.
4 CLINICAL PARTICULARS
4.1 Target Species
Cattle.
Each ml contains:
Active Substance:
Ivermectin 10 mg
Clorsulon 100 mg
4.2 Indications for use, specifying the target species
Ivomec Super Injection for Cattle is indicated for the treatment and control of the following parasites:
Gastrointestinal roundworms (adult and fourth-stage larvae):
Ostertagia spp. (including inhibited O. ostertagi)
Haemonchus placei
Trichostrongylus axei
T. colubriformis
Cooperia spp.
Bunostomum phlebotomum
Oesophagostomum radiatum
Strongyloides papillosus (adult only)
Nematodirus helvetianus (adult only)
N. spathiger (adult only)
Toxocara vitulorum
Trichuris spp. (adult only)
Lungworms (adult and fourth-stage larvae):
Dictyocaulus viviparus
Liver fluke (adult)
Fasciola hepatica
Eye worms (adult)
Thelazia spp.
Warbles (parasitic stages):
Hypoderma bovis
H. lineatum
Mange mites :
Psoroptes bovis
Sarcoptes scabiei var. bovis
Sucking lice :
Linognathus vituli
Haematopinus eurysternus
Solenopotes capillatus
Ivomec Super Injection for Cattle may also be used as an aid in the control of biting lice (Damalinia bovis) and the
mange mite Chorioptes bovis, but complete elimination may not occur.
Persistent Activity
When cattle have to graze on pasture contaminated with infective larvae of cattle nematodes, treatment with Ivomec
Super Injection for Cattle at the recommended dose rate can control re-infection with Haemonchus placei and Cooperia spp., acquired up to 14 days after treatment, Ostertagia ostertagi and Oesophagostomum radiatum acquired up to 21 days after treatment and Dictyocaulus viviparus acquired up to 28 days after treatment.
To obtain optimal benefit from the persistent activity of Ivomec Super Injection for Cattle in grazing animals, it is
recommended that calves which are set-stocked in the first grazing season should be treated 3, 8 and 13 weeks after the day of turn-out.
This can protect the animals from parasitic gastro-enteritis and lungworm disease throughout the
grazing season, provided they are set-stocked, all the calves are included in the programme and that no untreated cattle are added to the pasture.
Treated animals should always be monitored according to good husbandry practices.
4.3 Contraindications
This product is not to be used intramuscularly or intravenously.
This product is registered for use in cattle only. Do not use in other species as severe adverse reactions, including
fatalities, may occur.
Do not use in animals with known hypersensitivity to the active ingredients.
4.4 Special warnings for each target species
Details provided above apply.
See also points 4.2, 4.3 and 4.5.
Care should be taken to avoid the following practices because they increase the risk of development of resistance and could ultimately result in ineffective therapy:
- Too frequent and repeated use of anthelmintics from the same class, over an extended period of time.
- Underdosing, which may be due to underestimation of body weight, misadministration of the product, or lack of
calibration of the dosing device.
Suspected clinical cases of resistance to anthelmintics should be further investigated using appropriate tests (e.g. Faecal Egg Count Reduction Test). Where the results of the test(s) strongly suggest resistance to a particular anthelmintic, an anthelmintic belonging to another pharmacological class and having a different mode of action should be used.
4.5 Special precautions for use
Special precautions for use in animals
Divide doses in excess of 10 ml between different injection sites and use different sites to those used for other
parenteral medications
Swab septum before removing each dose.
Use dry sterile needle and syringe.
When using the 200 ml and 500 ml pack sizes, use only automatic syringe equipment.
For the 50 ml pack size, the use of a multidose syringe is recommended.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Do not smoke or eat while handling the product.
Wash hands after use.
Take care to avoid self-administration; the product may cause local irritation and/or pain at the site of injection.
4.6 Adverse reactions (frequency and seriousness)
Transitory discomfort has been observed in some cattle following subcutaneous administration.
A low incidence of soft tissue swelling at the injection site has been observed.
These reactions disappeared without treatment.
4.7 Use during pregnancy, lactation or lay
Ivomec Super Injection for Cattle is safe for use at any stage of pregnancy or lactation.
However, the product is not permitted for use in animals producing milk for human consumption, including pregnant animals intended to produce milk for human consumption. Ivomec Super Injection for Cattle will not affect the fertility of cows and bulls and can be given to all ages of animals including young calves.
4.8 Interaction with other medicinal products and other forms of interaction
No interactions have been identified with other products.
4.9 Amounts to be administered and administration route
Ivomec Super Injection for Cattle should be given only by subcutaneous injection at the recommended dosage level of 1 ml/50 kg bodyweight (based on a dosage level of 200 mcg ivermectin plus 2 mg clorsulon per kg bodyweight) under the loose skin in front of, or behind, the shoulder.
Divide doses greater than 10 ml between two injection sites.
To ensure administration of a correct dose, body weight should be determined as accurately as possible; accuracy of the dosing device should be checked.
A sterile 17 gauge ½ inch (15-20mm) needle is recommended.
Replace with a fresh sterile needle after every 10-12
animals or sooner if the needle becomes soiled.
When the temperature of the product is below 5oC, difficulty in
administration may be encountered due to increased viscosity.
Warming the product and injection equipment to about
15oC will greatly increase the ease with which the product can be injected.
Different injection sites should be used for other parenteral products.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
The administration of 25 ml Ivomec Super Injection for Cattle per 50 kg bodyweight (25 x the use level) resulted in
injection site lesion (including tissue necrosis, oedema, fibrosis and inflammation).
No other drug-related adverse reactions could be determined.
4.11Withdrawal Period(s)
Cattle intended for human consumption may not be slaughtered until 66 days after the last treatment.
Not permitted for use in animals producing milk for human consumption, including pregnant animals intended to
produce milk for human consumption.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
Pharmacotherapeutic group:
Endectocides
ATCvet code:
QP54AA51
5.1 Pharmacodynamic properties
Ivermectin
Ivermectin is a member of the macrocyclic lactone class of endectocides which have a unique mode of action.
Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite.
Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gammaaminobutyric
acid (GABA)
The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated
chloride channels, the macrocyclic lactones have a low affinity for other mammalian ligand-gated chloride channels
and they do not readily cross the blood-brain barrier.
Clorsulon
Clorsulon is rapidly absorbed into the circulating blood. Erythrocytes with bound drug as well as plasma are ingested
by Fasciola spp.. Adult Fasciola spp. are killed by clorsulon because of inhibition of enzymes in the glycolytic
pathway, which is their primary source of energy.
5.2 Pharmacokinetic properties
Maximum plasma concentration
After subcutaneous administration of 2 mg clorsulon and 0.2 mg ivermectin per kg bodyweight, the plasma profile
demonstrated the slow, steady absorption of ivermectin with peak plasma levels averaging 23 ng/ml around day 7 post dose.
In contrast, clorsulon appeared rapidly absorbed since the first sampling point, 8 hours post dose, had the highest
average residues, approximately 2 μg/ml.
Excretion: length of time and route
A dose rate of 2 mg clorsulon and 0.2 mg ivermectin per kg bodyweight was administered by subcutaneous injection.
For ivermectin, liver had the highest average residues, peaking on day 7 post dose at an average of 220 ppb. Depletion followed so that by days 28 and 35 the liver residues were 11 and 6 ppb respectively. Fat residues also peaked on day 7 at an average of 160 ppb. They decreased to 6 and 4 ppb by days 28 and 35. Muscle and kidney residues were negligible at 1 and 2 ppb respectively by day 28.
For clorsulon, kidney had the highest average residues of 0.54 ppm (540 ppb) on day 3 post dose. At the same time,
liver averaged 0.20 ppm, muscle averaged 0.06 ppm and fat averaged 0.02 ppm. Rapid depletion followed, resulting in average residues at or below the detection limit of 0.01 ppm by day 21 for all tissues.
In cattle receiving a single dose of tritium-labelled ivermectin (0.2-0.3 mg/kg bodyweight), analyses show that
composites of faeces collected during the first 7 days after dosing contained almost all the dosed radioactivity, only
about 1-2% being excreted in the urine. Analyses of the faeces showed that about 40-50% of the excreted radioactivity was present as unaltered drug. The remaining 50-60% was present as metabolites or degradation products, almost all of which were more polar than the ivermectin.
During the first 7 days following intrarumen administration of 7 mg/kg clorsulon to a 270 kg steer, about 90% of the
radiolabel in an administered dose was found in both the urine (25%) and the faeces (65%).
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Glycerol formal
Propylene Glycol
6.2 Incompatibilities
In the absence of compatability studies, this veterinary medicinal product must not be mixed with other veterinary medicinal products.
6.3 Shelf-life
Shelf-life of the veterinary medicinal product as packaged for sale: 5 years
Shelf-life after first opening the immediate packaging: 3 months
6.4 Special precautions for storage
Protect from direct sunlight.
Do not store above 250C.
6.5 Nature and composition of immediate packaging
Multiple-dose rubber-capped polyethylene bottles of 50 ml, 200 ml and 500 ml containing a sterile non-aqueous
solution.
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal products or waste materials
Studies indicate that when ivermectin comes in contact with the soil, it readily and tightly binds to the soil and becomes inactive.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product
should be disposed of in accordance with local requirements.
Do not contaminate lakes or streams as free ivermectin may adversely affect fish and certain water-borne organisms.
7 MARKETING AUTHORISATION HOLDER
Merial Animal Health Limited
Sandringham House
Harlow Business Park
Harlow, Essex
CM19 5TG
England
8 MARKETING AUTHORISATION NUMBER(S)
VPA 10857/038/001
9 DATE OF THE FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
30th September 2009
10 DATE OF REVISION OF THE TEXT
5th May 2010
Cattle Injectables
Injectables should be given according to the manufacturer’s instructions at the recommended injection site.
• Always use a clean, sterile syringe and needle. If using a multiple injection gun, ensure the needle is disinfected between injections, e.g. with an automatic sterilisation system.
• If the site to be injected is dirty, clean the skin and swab with an alcohol-impregnated wipe or cotton wool.
• Before injecting, check the expiry date and read the instructions of the product to be used. Some products need to be shaken before use.
• Use the correct-sized needle according to the size of the animal and site of injection.
• Ensure the animal is adequately restrained before attempting the injection.
• Take care to ensure it is given subcutaneously and not intramuscularly. Raise a fold of skin at the injection site (mainly neck but some are ear) recommended by the product manufacturer and inject carefully into the space created.
• If a large dose is to be delivered, it may be advisable to split the dose between two injection sites. After the injection, briefly massage the site to improve the dispersal of the injected material.
• Dispose of the needle and syringe in appropriate clinical waste and sharps containers.
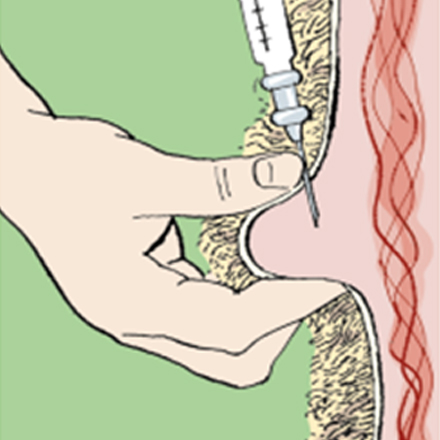
Ivomec Super injection is a broad spectrum anti parasitic for cattle. It is used for the treatment and control of gastro-intestinal nematodes, lungworms, adult liver fluke, eyeworms, warbles, mites and lice in beef and non lactating dairy cattle.
Active Ingredient: Ivermectin, Clorsulan
Target Species: Cattle
Treats and Controls: Gastro-intestinal roundworms, lungworms, adult liver fluke, eyeworms, warbles, mites and lice
Withdrawal Time: 66 days for animals intended for meat and offal, Not permitted for use on animals producing milk for human consumption.
Legal Status: POM
Here at Agridirect we have joined forces with DPD to ensure all packages are delivered promptly and safely to you. We ship to all mainland countries within the EU. Deliveries take place Monday to Friday excluding bank holidays. Once your order has been dispatched from our warehouse you will be notified by email. If there is a delay with your order for any reason you will be contacted immediately.
Due to Brexit we are temporarily unable to ship to the UK. Shipping to Northern Ireland will remain in place.
| Ireland (ROI & NI) | EU (Mainland Only) |
| 2-4 Working Days | 4-6 Working Days |
Some products have an extended delivery time, this is noted on the products.
| Country | Orders Under €100 | Orders Over €100 |
| Ireland (ROI & NI) | €7.99 | €0.00 |
| Austria | €39.99 | €32.00 |
| Belgium | €36.99 | €29.00 |
| Czech Republic | €39.99 | €32.00 |
| Denmark | €39.99 | €32.00 |
| Finland | €51.99 | €45.00 |
| France | €36.99 | €29.00 |
| Germany | €36.99 | €29.00 |
| Hungary | €43.99 | €37.00 |
| Italy | €49.99 | €42.00 |
| Luxenburg | €36.99 | €29.00 |
| Netherlands | €36.99 | €29.00 |
| Poland | €36.99 | €29.00 |
| Portugal | €51.99 | €45.00 |
| Slovakia | €43.99 | €37.00 |
| Slovenia | €43.99 | €37.00 |
| Spain | €49.99 | €42.00 |
| Sweden | €49.99 | €42.00 |
A selection of the products we sell are only licensed for sale within the Republic Of Ireland and can not be shipped outside of the country. These products are noted as only being available within the Republic of Ireland on the individual product pages.
There may be an addition charge on certain bulky items. This charge will be clearly marked on an applicable products and will be explained on the checkout page before payment has been made.
We’re sorry your purchase didn’t work out. But don’t worry; we have a great returns policy to help you out.
All purchases can be returned to us within 14 days of delivery and returned goods must be received within 14 days from the date you informed us of the return.
Purchases may be opened for inspection but must not be used and must be repackaged securely in the original packaging if you wish to return it.
If we discover goods have been used or there has been a loss in value of the goods due to damage to the goods, while in your care or whilst being returned to us, we will reduce the amount refunded, which may amount to the full cost of the product, to cover loss of value of goods.
All returns should be complete which includes boxes, manuals and accessories that may have been included with the order.
All returns must be packaged appropriately for shipping, we will not accept responsibility for damages or loss which occur during shipping of a return product.
We accept no responsibility for goods damaged or lost while in transit to us.
We have partnered with DPD to make your returns process easy and secure. simply follow the steps below and bring your package to an official DPD pickup point.
1) All returns must be accompanied with a fully filled out returns form which can be downloaded returns form PDF.
2) To print off your return label click DPD Returns page or visit www.dpd.ie/returns and follow the on-screen instructions. Make sure and use your order number as your reference.
3) Bring your package to a DPD pickup point. To find your nearest drop off point DPD pickup shops.
Once the returned product has been received into our warehouse and been fully inspected a refund will be issued.
If you choose not to use the DPD returns service we recommend that you use a method that can be tracked.
For the return of bulk products please contact us at sales@agridirect.ie
First off, if you have received a damaged electrical product from us, do not plug it in. Any electrical products that are plugged in are deemed ‘as used and accepted’ and are not accepted as returns. All damages must be reported to us via phone or email within 24hours of receipt of goods. Please ensure you check your items upon delivery.
How do I begin the returns process?
If you wish to begin the return process, please email us at sales@agridirect.ie and ensure the following information is included in your email. Your name, phone number, Order id, the item you wish to return, reason for return and if the product is damaged we require photos of the product.
Once you have sent us all required information a member of our team will assess your claim and will contact you as soon as possible. Please hold off on returning products until a member of our team has called you to confirm.
Once the returned product has been returned to us and fully inspected a refund will be issued.
Please Note: A typical timeline for a refund to show in your account is up to 10 working days from the date processed, depending on your bank.
Would you like to send this voucher to the recipient via email?
Yes No