This is a prescription-only medicine (POM).
A valid prescription is required to purchase this product.
Don’t have a prescription? Our team of vets is here to help.
Add to cart as normal and follow the steps. Available in Ireland only.
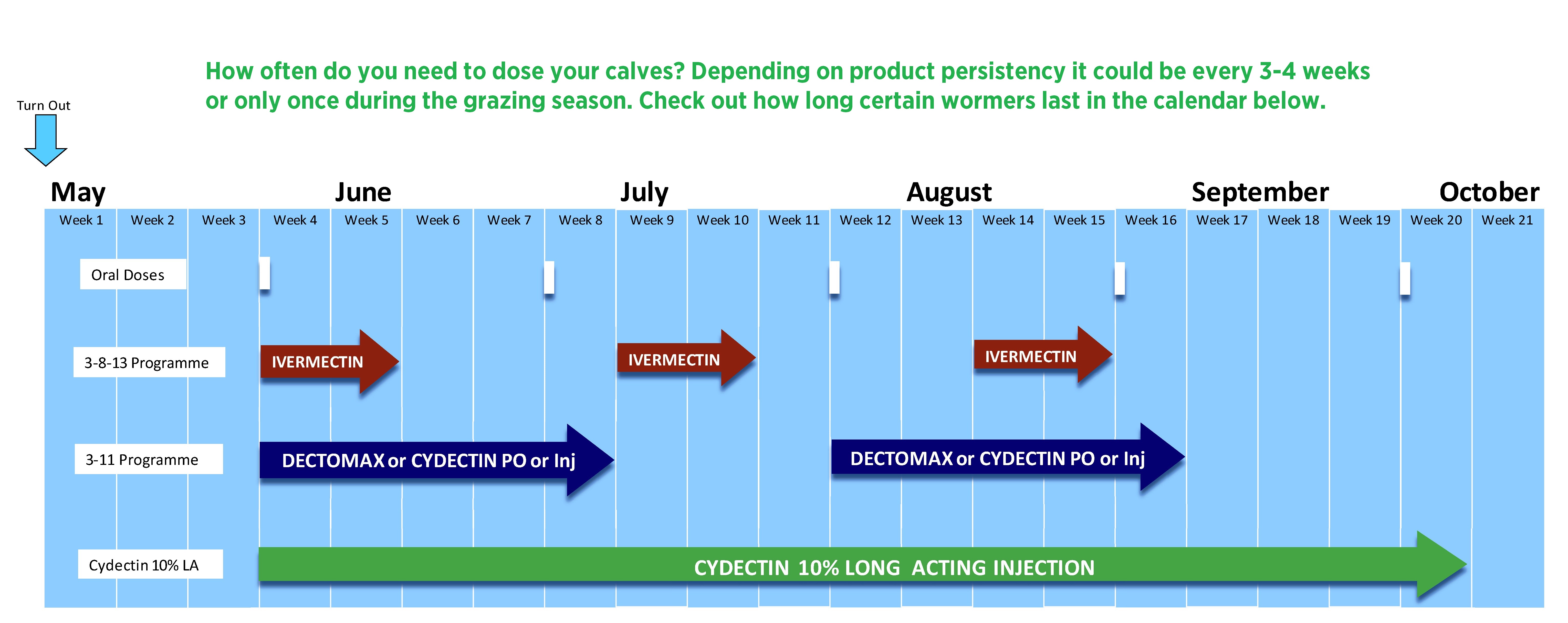
Cydectin 10% LA is a single dose long acting injection used for the treatment and control of both internal and external parasites. Cydectin 10% LA specifically targets mature and immature stomach & gut worms, lung worms, warbles, lice and mange mites. A single dose of Cydectin 10% LA's specially designed long acting formula can last an entire grazing season. This leads to both time and money being saved by not having to dose mid season.
Active Ingredient: Moxidectin
Target Species: Cattle
Treats and Controls: mature and immature stomach worms, lung worms, warbles, lice and mange mites
Administration Method: Subcutaneous injection (under the skin)
Withdrawal Time: 108 days for animals intended for meat and offal, Not permitted for use on animals producing milk for human consumption.
Dosage: 0.5 ml per 50 kg of bodyweight, do not use in animals less than 100 kg or over 500 kg in weight
| Body Weight | Dose Volume | Number of full doses per pack: |
| 50 ml | ||
| 100kg | 1 ml | 50 |
| 150kg | 1.5 ml | 33 |
| 200kg | 2 ml | 25 |
| 250kg | 2.5 ml | 20 |
| 300kg | 3 ml | 17 |
| 350kg | 3.5 ml | 14 |
| 400kg | 4 ml | 13 |
| 450kg | 4.5 ml | 11 |
| 500kg | 5 ml | 10 |
Always read the label and all enclosed information for Cydectin 10% LA before administering to animals!
| Signs and Effects of Infected Cattle |
|
Infection: Gut Worm Symptoms: Diarrhoea, decreased appetite, loss of weight Effects: Gutworm can cause severe damage to the stomach and small intestine which will cause parasitic gastroenteritis, this will not only negatively affect the health of the animal but will affect the profitability for the farmer. |
|
Infection: Lungworm Symptoms: Short, sharp cough that becomes worse with exercise, in severe cases the animal will have obvious difficulty breathing. Effects: Lung worm infections cause a high susceptibility to respiratory viruses and bacteria. Infected cattle are prone to contracting severe bronchial pneumonia which left untreated can lead to death. |
This product is only licensed for sale within the Republic of Ireland
Click here to Download Data Sheet
Health Products Regulatory Authority
Summary of Product Characteristics
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Cydectin 10 % LA Solution for Injection for Cattle
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml contains:
Active substance
Moxidectin 100 mg
Excipeints
Benzyl Alcohol (E1519) 70 mg
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection.
Clear yellow liquid.
4 CLINICAL PARTICULARS
4.1 Target Species
Cattle.
4.2 Indications for use, specifying the target species
In cattle weighing from 100 to 500 kg body weight, treatment and prevention of mixed infestations by the following gastro-intestinal nematodes, respiratory nematodes and certain arthropod parasites :
Adult and immature gastro-intestinal nematodes : .
Haemonchus placei .
Haemonchus contortus .
Ostertagia ostertagi (including inhibited larvae) .
Trichostrongylus axei .
Trichostrongylus colubriformis .
Nematodirus helvetianus (adults only) .
Nematodirus spathiger .
Cooperia surnabada .
Cooperia oncophora .
Cooperia pectinata .
Cooperia punctata .
Oesophagostomum radiatum .
Bunostomum phlebotomum (adults only) .
Chabertia ovina (adults only) .
Trichuris spp. (adults only)
Adult and immature respiratory tract nematode .
Dictyocaulus viviparus
Warble grubs (migrating larvae) .
Hypoderma bovis .
Hypoderma lineatum
Lice .
Linognathus vituli .
Haematopinus eurysternus .
Solenopotes capillatus . B
ovicolabovis (aid in control)
Mange mites .
Sarcoptes scabiei .
Psoroptes ovis .
Chorioptes bovis (aid in control)
The drug has a persistent action and protects cattle for a certain duration against infection or re-infection with the following parasites for the period indicated:
Species Protection period (days)
Dictyocaulus viviparus 120
Ostertagia ostertagi 120
Haemonchus placei 90
Oesophagostomum radiatum 150
Trichostrongylus axei 90
Linognathus vituli 133
The product is effective against Hypodermalarvae at the time of treatment but its persistent activity against Hypodermahas not been evaluated.
If the product is given before the end of the fly season complimentary treatment with a product effective against Hypoderma may be required.
Persistent efficacy periods have not been established for parasite species other than those included in the above list. Therefore, re-infection of animals on pasture contaminated by parasites other than these remains possible before the end of the 90 day minimum persistency period demonstrated for specific species.
4.3 Contraindications
Do not use in animals less than 100 kg bodyweight or greater than 500 kg.
Do not inject the product by intravascular route.
Intravascular injection may result in ataxia, paralysis, convulsions, collapse and death.
To prevent any intravascular injection, carefully follow the administration procedure described in item “Amounts to be administered and administration route”.
4.4 Special warnings for each target species
Care should be taken to avoid the following practices, because they increase the risk of development of resistance and could ultimately result in ineffective therapy:
- Too frequent and repeated use of anthelmintics from the same class, over an extended period of time;
- Under-dosing which may due to underestimation of body weight, misadministration of the product, or lack of calibration of the dosing device (If any).
- Suspected clinical cases of resistance to anthelmintics should be further investigated using appropriate tests (e.g. Faecal Egg Count Reduction Test).
Where the results of the test(s) strongly suggest resistance to a particular anthelmintic, an anthelmintic belonging to another pharmacological class and having a different mode of action should be used.
4.5 Special precautions for use
Special precautions for use in animals
In order to prevent abscesses, a strict aseptic technique is recommended.
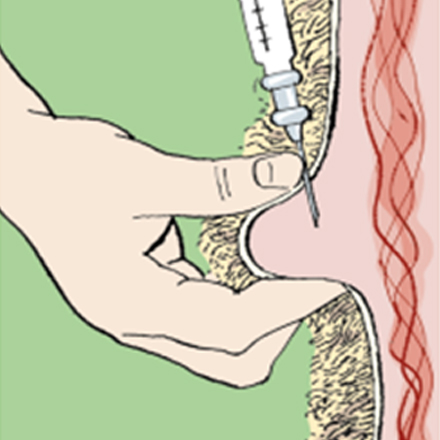
CYDECTIN 10% LA for Cattle has been formulated specifically for subcutaneous injection in dorsal surface of the ear of cattle and must not be given by any other route of administration or to any other species.
To avoid possible secondary reactions by the death of Hypodermalarvae in the spine or the oesophagus of animals, it is recommended to administer a product effective against Hypodermalarvae after the end of fly activity and before the larvae reach their resting sites.
Consult your veterinary surgeon on the correct timing of this treatment.
Immunity to nematodes depends on adequate exposure to infection.
Although not normally the case, circumstances could occur in which anthelmintic control measures might increase the vulnerability of cattle to re-infection.
Animals may be at risk towards the end of their first grazing season, particularly if the season is long, or in the following year if they move onto heavily contaminated pasture.
In such instances, further control measures may be necessary.
Special precautions to be taken by the person administering the veterinary medicinal product to animals Avoid direct contact with skin and eyes.
Wash hands after use.
Do not smoke, drink or eat while handling the product.
Take care to avoid self-injection.
Advice to Medical Practitioners in case of accidental self injection:
Treat symptomatically.
Other precautions regarding impact on the environment
Moxidectin fulfils the criteria for a (very) persistent, bioaccumulative and toxic (PBT) substance; therefore, exposure of the environment to moxidectin must be limited to the extent possible.
Treatments should be administered only when necessary and should be based on faecal egg counts or evaluation of the risk of infestation at the animal and/or herd level.
Like other macrocyclic lactones, moxidectin has the potential to adversely affect non-target organisms:
Faeces containing moxidectin excreted onto pasture by treated animals may temporarily reduce the abundance of dung feeding organisms.
Following treatment of cattle with the product, levels of moxidectin that are potentially toxic to dung fly species may be excreted over a period more than 4 weeks and may decrease dung fly abundance during that period.
It has been established in laboratory tests that moxidectin may temporarily affect dung beetle reproduction; however, field studies indicate no long-term effects.
Nevertheless, in case of repeated treatments with moxidectin (as with products of the same anthelmintic class) it is advisable not to treat animals every time on the same pasture to allow dung fauna populations to recover.
Moxidectin is inherently toxic to aquatic organisms including fish.
The product should be used only according to the label instructions. Based on the excretion profile of moxidectin when administered as the injectable formulation, treated animals should not have access to watercourses during the 10 days after treatment.
4.6 Adverse reactions (frequency and seriousness)
On rare occasions, immediate or delayed swelling can be observed at the injection site, these swellings may further develop into abscesses (approx. 1% of cases).
The frequency of injection site swellings tends to be higher in the heavier animals.
These side effects generally disappear without treatment, within 14 days after administration, some may persist for up to 5 weeks in a number of animals (<5%) and in very rare occasions longer.
On rare occasions, depression and ataxia can be observed after injection.
In case of hypersensitivity reactions, a symptomatic treatment should be applied.
The frequency of adverse reactions is defined using the following convention:
-very common (more than 1 in 10 animals displaying adverse reaction(s))
-common (more than 1 but less than 10 animals in 100 animals treated)
-uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
-rare (more than 1 but less than 10 animals in 10,000 animals treated)
-very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
4.7 Use during pregnancy, lactation or lay
Can be used during pregnancy. However, note 4.3. Contra-Indications.
4.8 Interaction with other medicinal products and other forms of interactions
The effects of GABA agonists are increased by moxidectin.
4.9 Amounts to be administered and administration route
Dosage is 0.5 ml/50 kg bodyweight, equivalent to 1.0 mg moxidectin/kg bodyweight, given by a single subcutaneous injection in the ear using an 18 gauge, 25 – 40 mm hypodermic needle.
The 50ml vial stoppers must not be broached more than 20 times.
Use automatic syringe equipment for the 200 ml vial.
Shake well before use.
To ensure administration of a correct dosage, body weight should be determined as accurately as possible; accuracy of the dosing should be checked.
The injection should be given subcutaneously in the loose tissues on the dorsal surface of the ear, just distal to the distal edge of the auricular cartilage.
The dorsal (outer) surface of the ear should first be cleansed with antiseptic and allowed to briefly air dry.
Palpate the edge of the auricular cartilage closest to the head, on the dorsal (hairy) surface of the ear.
From this landmark, taking care to avoid blood vessels (artery, vein), the needle should be inserted subcutaneously starting at a point approximately 3 to 3.5 cm distal to this edge (away from the head), and directed towards the base of the ear, and the needle advanced to the hub.
At this point, gently aspirate the syringe to confirm that the needle is not in a blood vessel.
Upon injection, the resulting depot should reside just distal to the edge of the auricular cartilage.
Following administration, the needle is withdrawn from the skin as pressure is applied for several seconds with the thumb at the point of insertion.
Due to the long lasting protection against Dictyocaulus viviparus and the stomach worms, Ostertagia ostertagi and Haemonchus placei, a single treatment with the formulation at turn-out helps control parasitic bronchitis (lungworm) and parasitic gastro-enteritis throughout the grazing season by reducing the build-up of infective larvae on pasture associated with these parasites.
For best results the injection should be given to each calf of target weight to be grazed together immediately prior to being turned out to pasture.
Animals should be set stocked throughout the grazing season or moved to a pasture which has not been grazed by other cattle earlier in the season.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Reactions at the injection site have to be expected more frequently and severe depending on the injected volume. Systemic signs of overdoses are consistent with the mode of action of moxidectin.
These signs are manifested as transient salivation, depression, drowsiness and ataxia 24 to 36 hours post-treatment. The systemic signs usually disappear within 36 to 72 hours without treatment.
At doses >3 times the recommended dose divided on both ears, the systemic signs included recumbency, muscle tremor, ruminal tympany and dehydration, which were resolved after treatment with fluids.
The systemic signs can last for a few days to ten days.
There is no specific antidote.
4.11 Withdrawal period(s)
Meat and offal: 108 days.
Milk: Not permitted for use in lactating animals producing milk for human consumption or industrial purposes or within 80 days of expected parturition.
The withdrawal period is based solely on a single injection at the ear site of injection.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
ATCvet Code
QP 54 AB 02
Pharmacotherapeutic group:
Endectocides
5.1 Pharmacodynamic properties
Moxidectin is an endectocide active against a wide range of internal and external parasites and is a second generation macrocyclic lactone of the milbemycin family.
Moxidectin interacts with GABA receptors and chloride channels.
The net effect is to open the chloride channels on the postsynaptic junction to allow the inflow of chloride ions and induce an irreversible resting state.
This results in flaccid paralysis and eventual death of parasites exposed to the drug.
5.2 Pharmacokinetic particulars
Moxidectin is absorbed following subcutaneous injection with maximum blood concentrations being achieved 24 to 48 hours post injection.
The drug is distributed throughout the body tissues but due to its lipophilicity it is concentrated mainly in the fat.
The depletion half life in fat is 26 – 32 days.
Moxidectin undergoes limited biotransformation by hydroxylation in the body.
The only significant route of excretion is the faeces.
5.3 Environmental properties
Moxidectin fulfils the criteria for a (very) persistent, bioaccumulative and toxic (PBT) substance. In particular, in acute and chronic toxicity studies with algae, crustaceans and fish, moxidectin showed toxicity to these organisms, yielding the following endpoints:
Organism EC50 NOEC
Algae S. capricornutum >86.9 μg/l 86.9 μg/l Crustaceans (Water fleas) Daphnia magna (acute) 0.0302 μg/l 0.011 μg/l
Daphnia magna (reproduction) 0.0031 μg/l 0.010 μg/l
Fish O. mykiss 0.160 μg/l Not determined
L. macrochirus 0.620 μg/l 0.52 μg/l
P. promelas (early life stages) Not applicable 0.0032 μg/l
Cyprinus carpio 0.11 μg/l Not determined
EC50: the concentration which results in 50% of the test species individuals being adversely affected, i.e. both mortality and sub-lethal effects.
NOEC: the concentration in the study at which no effects are observed.
This implies that when allowing moxidectin to enter water bodies, this may have a severe and lasting impact on aquatic life. To mitigate this risk, all precautions for use and disposal must be adhered to.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Benzyl alcohol (E1519)
Sorbitan Monooleate (Crill 4HP)
Propylene Glycol
Dicaprylate/Dicaprate
6.2 Major incompatibilities
In the absence of compatibility studies, this veterinary medicinal product must not be mixed with other veterinary medicinal products.
6.3 Shelf-life
Shelf life of the veterinary medicinal product as packaged for sale: 3 years.
Shelf life after first opening the immediate packaging: 28 days.
6.4 Special precautions for storage
Do not store above 25°C.
Protect from light.
6.5 Nature and composition of immediate packaging
Nature of the primary container:
Flurotec coated chlorinated butyl rubber stopper
Aluminium flip off seal (50 ml vial)
Aluminium seal (200 ml)
Presentations to be sold and identification numbers:
Box containing 1 vial of 50ml size Box 1 vial of 200ml size
6.6 Special precautions for the disposal of unused veterinary medicinal products or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste material derived from such veterinary medicinal products should be disposed of in accordance with local requirements.
Do not contaminate watercourses with the product.
Extremely Dangerous for fish and aquatic organisms.
7 MARKETING AUTHORISATION HOLDER
Zoetis Belgium S.A.
2nd Floor,
Building 10
Cherrywood Business Park
Loughlinstown
Co Dublin
Ireland
8 MARKETING AUTHORISATION NUMBER(S)
VPA10387/015/001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 21st October 2005
Date of last renewal: 17th January 2010
10 DATE OF REVISION OF THE TEXT
September 2018
Cattle Injectables
Injectables should be given according to the manufacturer’s instructions at the recommended injection site.
• Always use a clean, sterile syringe and needle. If using a multiple injection gun, ensure the needle is disinfected between injections, e.g. with an automatic sterilisation system.
• If the site to be injected is dirty, clean the skin and swab with an alcohol-impregnated wipe or cotton wool.
• Before injecting, check the expiry date and read the instructions of the product to be used. Some products need to be shaken before use.
• Use the correct-sized needle according to the size of the animal and site of injection.
• Ensure the animal is adequately restrained before attempting the injection.
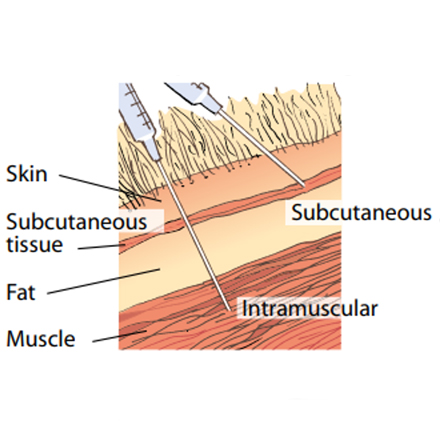
• Take care to ensure it is given subcutaneously and not intramuscularly. Raise a fold of skin at the injection site (mainly neck but some are ear) recommended by the product manufacturer and inject carefully into the space created.
• If a large dose is to be delivered, it may be advisable to split the dose between two injection sites. After the injection, briefly massage the site to improve the dispersal of the injected material.
• Dispose of the needle and syringe in appropriate clinical waste and sharps containers.
Cydectin 10% LA is a single dose long acting injection used for the treatment and control of both internal and external parasites. Cydectin 10% LA specifically targets mature and immature stomach & gut worms, lung worms, warbles, lice and mange mites.
Active Ingredient: Moxidectin
Target Species: Cattle
Treats and Controls: mature and immature stomach worms, lung worms, warbles, lice and mange mites
Withdrawal Time: 108 days for animals intended for meat and offal, Not permitted for use on animals producing milk for human consumption.
Legal Status: POM
Here at Agridirect we have joined forces with DPD to ensure all packages are delivered promptly and safely to you. We ship to all mainland countries within the EU. Deliveries take place Monday to Friday excluding bank holidays. Once your order has been dispatched from our warehouse you will be notified by email. If there is a delay with your order for any reason you will be contacted immediately.
Due to Brexit we are temporarily unable to ship to the UK. Shipping to Northern Ireland will remain in place.
| Ireland (ROI & NI) | EU (Mainland Only) |
| 2-4 Working Days | 4-6 Working Days |
Some products have an extended delivery time, this is noted on the products.
| Country | Orders Under €100 | Orders Over €100 |
| Ireland (ROI & NI) | €7.99 | €0.00 |
| Austria | €39.99 | €32.00 |
| Belgium | €36.99 | €29.00 |
| Czech Republic | €39.99 | €32.00 |
| Denmark | €39.99 | €32.00 |
| Finland | €51.99 | €45.00 |
| France | €36.99 | €29.00 |
| Germany | €36.99 | €29.00 |
| Hungary | €43.99 | €37.00 |
| Italy | €49.99 | €42.00 |
| Luxenburg | €36.99 | €29.00 |
| Netherlands | €36.99 | €29.00 |
| Poland | €36.99 | €29.00 |
| Portugal | €51.99 | €45.00 |
| Slovakia | €43.99 | €37.00 |
| Slovenia | €43.99 | €37.00 |
| Spain | €49.99 | €42.00 |
| Sweden | €49.99 | €42.00 |
A selection of the products we sell are only licensed for sale within the Republic Of Ireland and can not be shipped outside of the country. These products are noted as only being available within the Republic of Ireland on the individual product pages.
There may be an addition charge on certain bulky items. This charge will be clearly marked on an applicable products and will be explained on the checkout page before payment has been made.
We’re sorry your purchase didn’t work out. But don’t worry; we have a great returns policy to help you out.
All purchases can be returned to us within 14 days of delivery and returned goods must be received within 14 days from the date you informed us of the return.
Purchases may be opened for inspection but must not be used and must be repackaged securely in the original packaging if you wish to return it.
If we discover goods have been used or there has been a loss in value of the goods due to damage to the goods, while in your care or whilst being returned to us, we will reduce the amount refunded, which may amount to the full cost of the product, to cover loss of value of goods.
All returns should be complete which includes boxes, manuals and accessories that may have been included with the order.
All returns must be packaged appropriately for shipping, we will not accept responsibility for damages or loss which occur during shipping of a return product.
We accept no responsibility for goods damaged or lost while in transit to us.
We have partnered with DPD to make your returns process easy and secure. simply follow the steps below and bring your package to an official DPD pickup point.
1) All returns must be accompanied with a fully filled out returns form which can be downloaded returns form PDF.
2) To print off your return label click DPD Returns page or visit www.dpd.ie/returns and follow the on-screen instructions. Make sure and use your order number as your reference.
3) Bring your package to a DPD pickup point. To find your nearest drop off point DPD pickup shops.
Once the returned product has been received into our warehouse and been fully inspected a refund will be issued.
If you choose not to use the DPD returns service we recommend that you use a method that can be tracked.
For the return of bulk products please contact us at sales@agridirect.ie
First off, if you have received a damaged electrical product from us, do not plug it in. Any electrical products that are plugged in are deemed ‘as used and accepted’ and are not accepted as returns. All damages must be reported to us via phone or email within 24hours of receipt of goods. Please ensure you check your items upon delivery.
How do I begin the returns process?
If you wish to begin the return process, please email us at sales@agridirect.ie and ensure the following information is included in your email. Your name, phone number, Order id, the item you wish to return, reason for return and if the product is damaged we require photos of the product.
Once you have sent us all required information a member of our team will assess your claim and will contact you as soon as possible. Please hold off on returning products until a member of our team has called you to confirm.
Once the returned product has been returned to us and fully inspected a refund will be issued.
Please Note: A typical timeline for a refund to show in your account is up to 10 working days from the date processed, depending on your bank.
Would you like to send this voucher to the recipient via email?
Yes No